
So the electron configuration of calcium (Ca) which has 20 electrons, can be written as: 4s².
#AS ELEMENT CONFIGURATION PLUS#
The electron configuration can be seen as consisting of the core electrons, which is equal to the configuration of the most recent noble gas, plus the valence (outer electron) configuration of the element. So scientists will often use an abbreviated notation. Sometimes, writing out the entire notation can be time-wasting, especially for atoms with a lot of electrons. “Protons give an atom its identity, electrons its personality.” - Bill Bryson Adding up all the superscript numbers gives us 5, and boron has 5 electrons. Boron (B) has an electron configuration 1s☢s☢p¹. One way to check if the notation is correct for a given element is to see if the sum of the exponents in the notation equals the number of electrons in an atom of that element. The electron configuration follows a periodic order, where lower-level shells are filled in before higher-level shells. Examine the pattern that arises with the first 10 elements: Since one knows the order in which electrons fill in orbitals and one knows the number of electrons of each element, one can construct a unique electron configuration notation for each element. The tendency for an electron to fill in its lower level orbitals before higher-level ones is sometimes referred to as the Aufbau principle. So, an atom will fill the 1s orbital before filling the 2s orbital, the 2s orbital before the 2p orbital, the 2p orbital before the 3s orbital, and so on. An atom will fill all the s orbitals on a given shell before filling in any p orbitals and fill any p orbitals before filling in d orbitals. In general, atoms will completely fill a lower level orbital before filling a higher one. The red diagonal lines in the above chart represent the sequence in which an atom will fill its orbitals. In fact, one can figure out the electron configuration notation for any element by recognizing the pattern in which electrons fill in orbitals. The notation for carbon (C) is 1s☢s☢p² as carbon has 2 electrons in the s orbital of the first shell, two electrons in the s orbital of shell 2, and 2 electrons in the p orbital of shell 2. Similarly, the notation for helium (He) is 1s² because helium has 2 electrons in the s orbital of its first shell. This notation means that hydrogen has 1 electron in its s orbital on the first shell. Hydrogen’s (H) electron configuration notation is 1s¹. The amount of electrons in each orbital is represented as a superscript.Īs an example, consider hydrogen. Thus, an s orbital can hold a total of two electrons, a p orbital can hold a total of 6 electrons, a d orbital 10 and an f orbital 14.įor any given element, that element’s electron configuration can be represented as some sequence of shell labels and orbital labels. The total number of electrons that can fit a given orbital is determined by 2(2ℓ+1). The values ℓ = 0, 1, 2, 3 correspond to the orbitals s, p, d, and f, respectively. This number describes the shape of the orbital. Each orbital (s, p, d, f) has a number associated with it, called its azimuthal quantum number, sometimes referred to as ℓ. 1s refers to the s orbital on the first shell, 3p refers to the p orbital on the 3rd shell, etc. Accordingly, shell 1 can hold a total of 2 electrons, shell 2 can hold a total of 8 electrons, shell three a total of 18, etc.Įach letter (s, p, d, f) corresponds to a particular orbital (sometimes called subshell). For any electron shell n, that shell can hold a total of 2 n² electrons. So, 1 refers to the first shell, 2 the second shell, and so on. In this chart, the numbers (1, 2, 3,…) are referred to as the principal quantum number, referred to as n, which corresponds to an electron shell. An electron configuration chart of the elements shows the periodicity of the electron structure across the elements. In general, electrons will completely fill lower-level orbitals in lower level orbitals first before moving on to higher orbitals.Įlectrons will fill orbitals in a specific order. Atoms will fill the orbitals in their shells with electrons until they reach a stable configuration. Each orbital only has a finite number of spots for electrons. These shells, in turn, have orbitals - regions of the shell where electrons inhabit.
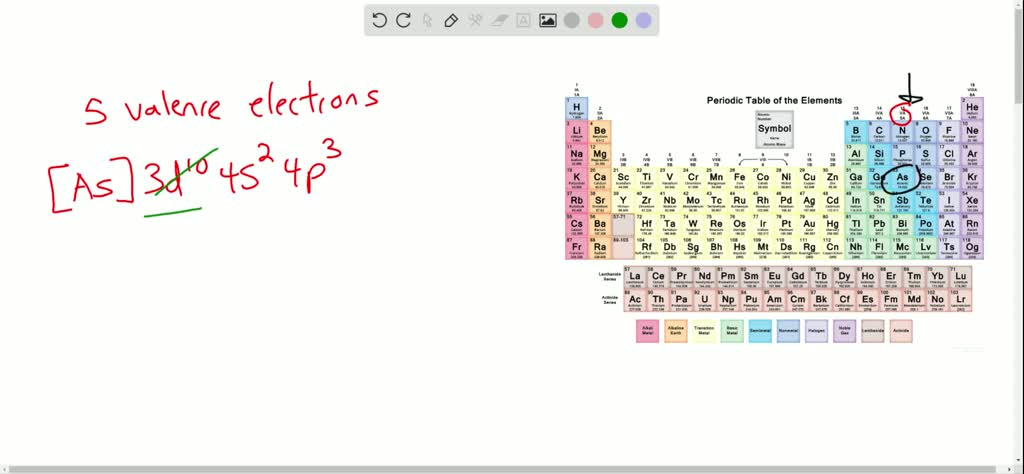
“With chemicals, it’s shoot first and ask questions later.” - Al Meyerhoff What Are Electrons?Įlectrons exist in shells that surround the nucleus of an atom. An electron configuration chart gives information about the orbital structure of the elements and how those orbitals are filled with electrons. An electron configuration chart is a tabular representation of patterns in the electron configuration of elements as one goes down the periodic table of elements.


 0 kommentar(er)
0 kommentar(er)
